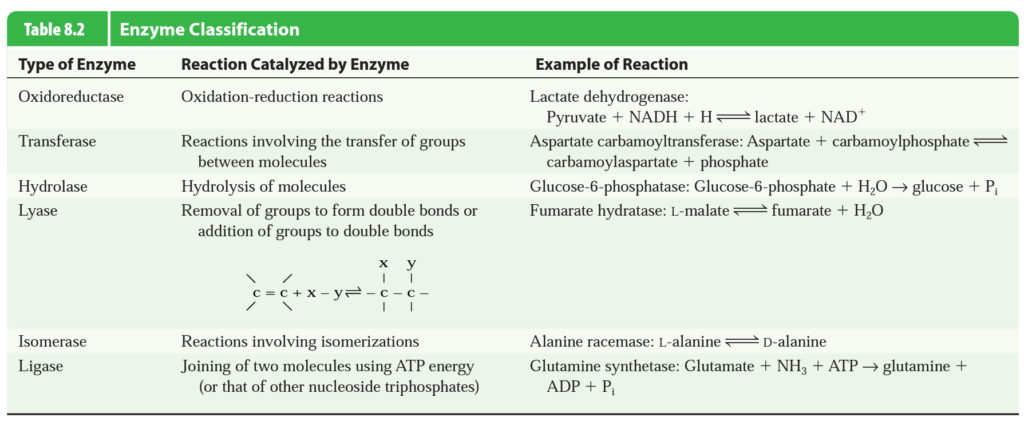
Structure And Classification Of Enzyme
Enzymes may be defined as protein catalysts that have great specification towards reaction catalyzed and the molecules acted on. A catalyst is a substance that can change the rate of reaction by its presence, however, they themselves does not involve in the reaction. Thus we can say enzymes speed up the rate of a bio-chemical reaction (cellular reactions). The reacting molecules are called substrates and substance formed are called products. Here, enzymes acts as catalyst to manipulate the rate of reaction without reacting to substrate.
Most of the enzyme those involved in bio-chemical reactions are made up of proteins. However, some enzymes content a non-protein component in them, usually called a cofactor. In such enzymes, protein part is termed as apoenzyme, and non-protein part is termed as cofactor. The complete enzyme consisting of apoenzyme and cofactor is called holoenzyme.
On the bases of how cofactor attached to the protein part of enzyme they can be devided or named seperately. If the cofactor is firmly attached to the apoenzyme it is a prosthetic group. If the cofactor is loosely attached to the apoenzyme and can dissociate from the protein after products have been formed, it is called a coenzyme.
Enzymes usually are named in terms of the substrates they act on the type of reaction catalyzed. For example, lactate dehydrogenase (LDH) removes hydrogens from lactate.
All enzymes are proteins but all proteins are not enzyme.
The Mechanism Of Enzyme Reaction
To find out how enzymes manipulate the rate of reaction we need to look reaction at its molecular level. It is important to know that any enzyme can increase the rate of reaction without its own consumption in the reaction. i.e. enzyme can increase the rate of reaction but do not alter their equilibrium constants. If a reaction is endergonic, the presence of enzyme will not shift its equilibrium so that more products can be formed. Enzymes simply speed up the rate at which a reaction proceeds towards its final equilibrium.
To understand activity of enzyme in a reaction we should look the molecular mechanism of the reaction.
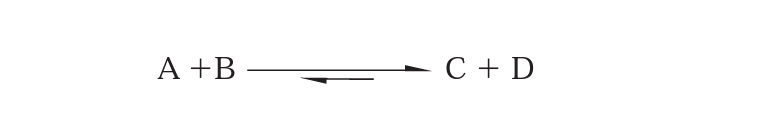
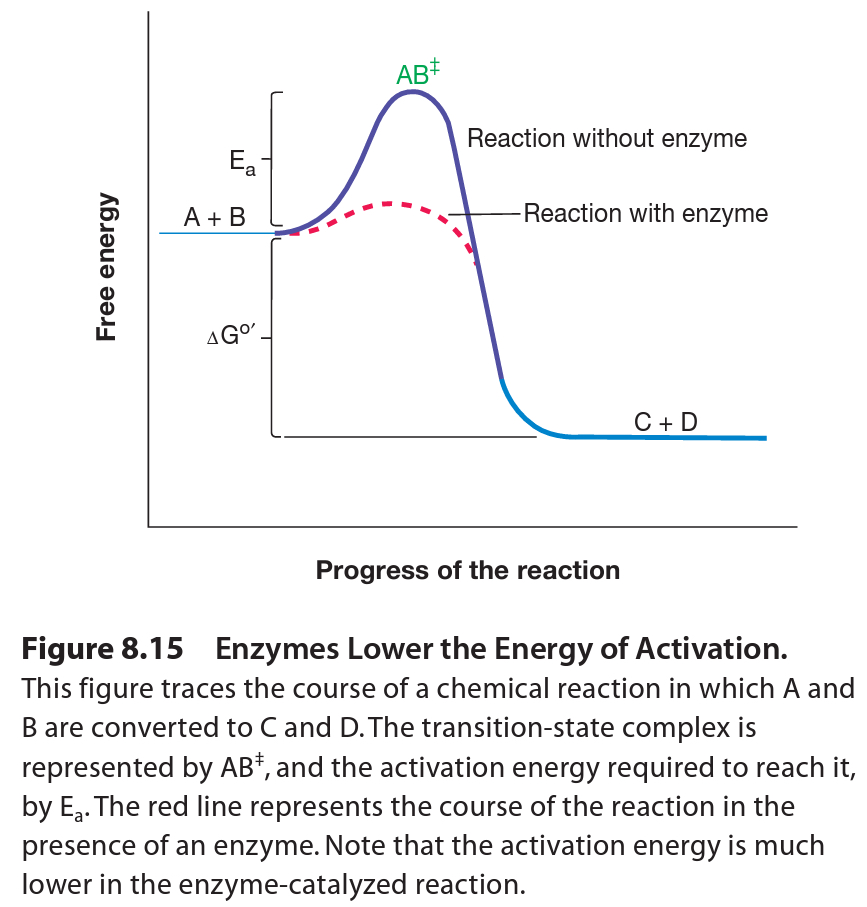
When molecules A and B approach each other to react, they form a transition-state complex, which resembles both the substrate and the products. Activation energy is required to bring the reacting molecules together in the correct way to reach the transition state. The transition-state complex can then resolve to yield the products C and D. the difference in free energy level between reactants and products is delta G. thus the equilibrium in our example will lie towards the products because (delta) G is negative. (i.e. products are at low energy level then reactants).
Thus we can say, A and B will not be converted to C and D if they are not supplied with an amount of energy equivalent to the activation energy. Enzymes accelerate reactions by lowering the activation energy; therefore more substrate molecules will have sufficient energy to come together and form products.
How Enzyme Lower The Activation Energy?
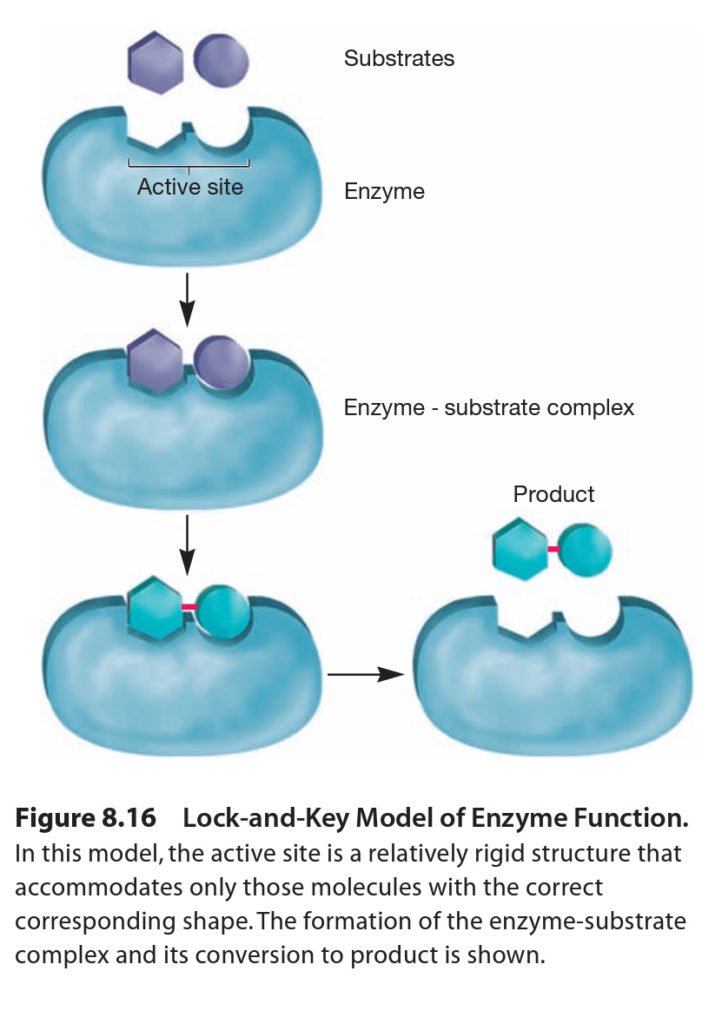
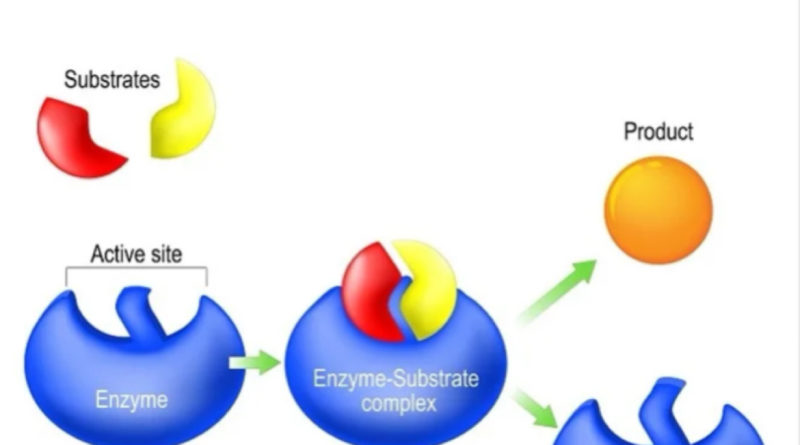
Enzymes bring substrates together at a specific place on their surface called the active site or catalytic site to form and enzyme-substrate complex. There are two methods through which enzyme can interact to its substance. Enzyme may be rigid and shaped to precisely fit the substrate so that the correct substrate binds specifically and is positioned properly for reaction. This mechanism is referred to as the lock-and-key model.
An enzyme may also change shape when it binds the substrate so that the active site surrounds and precisely fits the substrate. This has been called the induced fit model and is used by hexokinase and many other enzymes.
The formation of enzyme-substrate complex can lower the activation energy in many ways. For example, by bringing the substrate together at many sites, the enzyme is infect, concentrating substrate, and speeding up the reaction.