Clostridium Tetani
Cl. tetani is the causative organism of tetanus, a disease which is characterized by tonic muscular spasms, usually commencing at the site of infection and in all but the mildest cases becoming generalized, involving the whole of the somatic muscular system.
Nicolaier (1884), studying the disease, suggested that the manifestations of tetanus were due to a strychnine like poison produced by the bacillus multiplying locally.
The roel of bacillus in tetanus was furnished by Kitasato (1889) who isolate the bacillus in pure culture and reproduced the disease in animals by inoculation of pure cultures.
Cl. tetani is widely distributed in soil and in the intestines of human beings and animals. It is ubiquitous and has been recovered from wide variety of other sources like street and hospital dust, cotton wool, plaster of paris, bandages, catgut, talc,clothing etc. it may occur apparently harmless contaminant in wounds.
Morphology
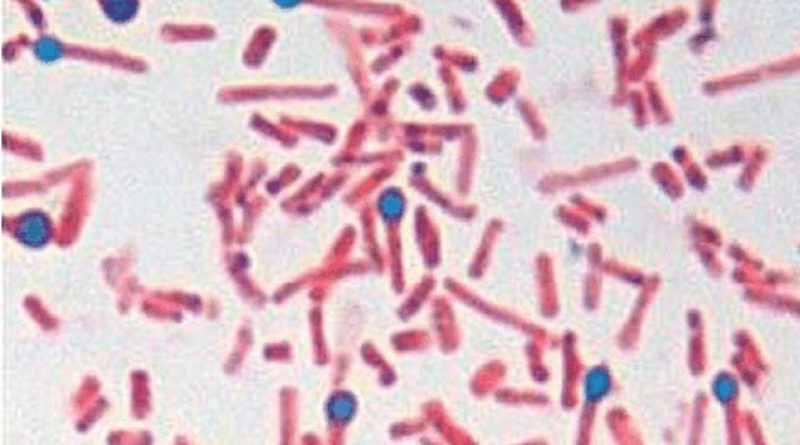
It is a Gram positive, slender bacillus, about 4 – 8 micrometer though there may be considerable variation in length. It has a straight axis, parallel sides and rounded ends. It is found singly and occasionally in chains.
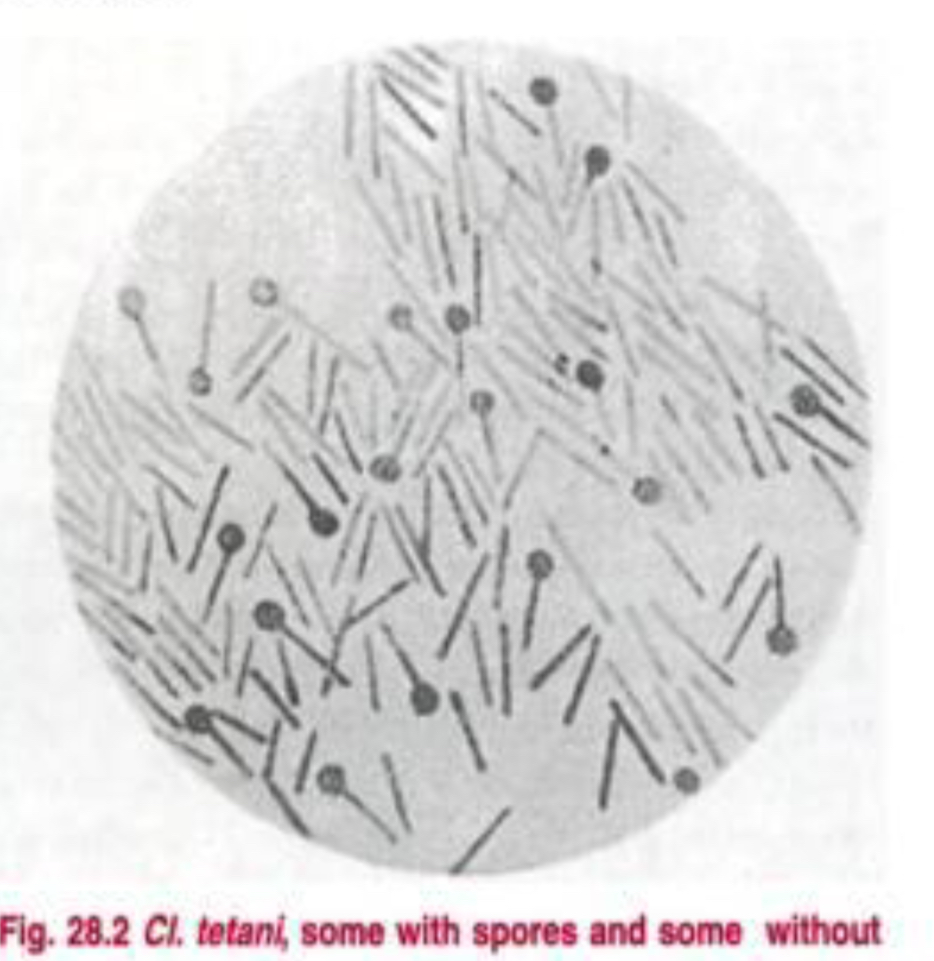
The spores of Cl. tetani are spherical, terminal and bulging, giving the bacillus the characteristic ‘drumstick’ appearance.
Young culture of Cl. tetani are strongly Gram positive but older cells show variable staining and may even be Gram negative.
Cultural Characteristics
Cl. tetani is obligatory anaerobe and grow only in the absence of oxygen. The optimum temperature of its growth is 37 C and pH 7.4. it can be grown on ordinary media. Its growth can be improved by blood and serum in culture media but not by glucose. Surface colonies are difficult to obtain as the growth has a marked tendency to swarm over the surface of the agar, especially if the medium is moist.
If the water of condensation at the bottom of a slope of nutrient agar is inoculated with the mixed cell culture, after incubation anaerobically for 24 hours, subcultures from the top of the tube will yield a pure growth of the tetanus bacillus (Filders’ technique).
It grows well in Robertson’s cooked meat broth, with turbidity and some gas formation. The meat is not digested but is turned blank on prolonged incubation.
On blood agar alpha hemolysis is produced, which later develops into beta hemolysis, due to the production of hemolysin (tetanolysin).
Biochemical Reactions
Cl. tetani has feeble proteolytic but no saccharolytic property. It does not attack any sugar. It forms indole. It is MR and VP negative. H2S is not formed. Nitrates are not reduced. Gelatin liquefaction occurs very slowly. A greenish fluorescence is produced on media containing neutral red (as on MacConkey’s medium).
Resistance
Most of the tetanus spores are killed by boiling for 10 – 15 minutes but some strains’ spores resist boiling for up to three hours. For destruction of spore it should be autoclave at 121 C for 20 minutes.
Spores are able to survive in soil for years, and are resistant to most antiseptics. They are not destroyed by 5% phenol or 0.1% mercuric chloride solution in two weeks or more. Iodine (1% aqueous solution) and hydrogen peroxide (10 volumes) kill the spores within a few hours.
Classification
Ten serological types have been recognized based on agglutination (types I to X). type VI contains no flagellated strains. All other types possess type specific flagellar antigens. All the type produce same toxin, which can be neutralized by antitoxin produced against any one type.
Toxins
Cl. tetani produces at least two distinct toxins – a hemolysin (tetanolysin) and a powerful neurotoxin (tetanospasmin). A third toxin, a nonspasomogenic, peripherally active neurotoxin, has been identified. It is not known whether this plays any role in the pathogenesis of tetanus.
Pathogenicity
Cl. tetani has little invasive power. Washed spores injected into experimental animals do not germinate and are destroyed by phagocytes. Germination and toxin production occur only if favorable conditions exist, such as reduced O-R potential, devitalised tissues, foreign bodies or concurrent infection.
The toxin produced locally is absorbed by the motor nerve endings and transported to the central nervous system intraxonally.
Laboratory Diagnosis
The diagnosis of tetanus should always be made on clinical grounds. Laboratory tests only help in confirmation.
Laboratory diagnosis may be made by the demonstration of Cl. tetani by microscopy, culture or by animal inoculation.
Microscopy is not reliable and the demonstration of the typical ‘drumstick’ bacilli in wounds in itself is not diagnostic of tetanus. The bacilli may be present in some wounds without tetanus developing.
Prophylaxis
Tetanus is a preventable disease. As the spores are ubiquitous, wound contamination is unavoidable. The disease id due to the action of the toxin. Therefore the obvious and most dependable immunity by active immunization by routine immunization of children and booster doses were appropriate.
The nature of prophylaxis depends largely on they type of the wound and the immune status of the patient. The available methods of prophylaxis are (1) surgical attention; (2) antibiotics and (3) immunization – passive, active or combined.

Reference: The text book of Microbiology