Ziehl Neelsen staining method
Ziehl Neelsen staining method is commonly used for staining of acid fast bacteria. In Mycobacterium and some species of Nocardia, the acid fastness property is correlated with their high lipid content because of this it is difficult to stain them. Hence for staining of these bacteria heating with strong dye is required. Once the bacteria are stained, it is difficult to deodorize them even with acid and alcohol. Non acid fast bacteria lose primary dye and take counterstain.
Requirements
- Trypticase soy broth for Mycobacterium culture.
- Carbon fuchsia
- Methylene blue
- Deodorizing solvent
- Bunsen burner
- Inoculation loop
- Glass slide
- Staining tray
- Microscope
Preparation of reagents:
Carbol fuchsin stain:
- Basic fuchsin 0.3g
- Ethanol (95%) 10 ml
- Phenol (heat melted crystals) 5 ml
The basic fuchsin dissolved in ethanol, and then in phenol in water. Mix them and keep for several days. Filter it before use.
Deodorizing solvent
- Ethanol (95%) 97 ml
- Hydrochloric acid (conc.) 3 ml
Counter stain
- Methylene blue chloride 0.3 g
- Distilled water 100 ml
Procedure
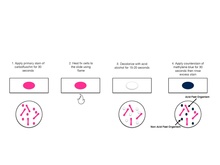
- Place the slide with an air dried and heat fixed smear on a hot plate until the steam rises with carbol fuchsin stained smear. Keep the preparation moist with stain and steaming for 5 min. Don’t boil it.
- Wash the film with a gentle stream of water until no color appears from effluent.
- Decolourise with HCl (3%) and immediately wash with tap water.
- Now, counterstain with malachite green (1%) for 20 to 30 seconds and wash with tap water.
Results
Observe under oil immersion objective of microscope. Acid fast bacteria appear Red, and non acid fast appear blue.
Reference: RC Dubey – Practical Microbiology